1999-2003: BONE INJECTION GUN (B.I.G.)
Bone Injection Gun / Intraosseous Access
The Bone Injection Gun, or “B.I.G.”, intraosseous (IO) device was approved for use in the United States by the FDA (Food & Drug Administration) circa 1999 for adults and circa 2003 for pediatric patients, but was used extensively for several years prior elsewhere in the world.
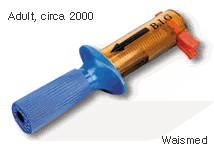

Somewhat ironically, the B.I.G. bore no resemblance to it’s mnemonic. It was not big, rather it was quite compact in size. (See accompanying pictures) Simply put, the B.I.G. was an intraosseous access device which afforded fast, definitive pediatric and adult IO access in a field setting, with little difficulty and proper training. It was also unlike other IO devices in that it was not limited to the sternal or tibial locations.
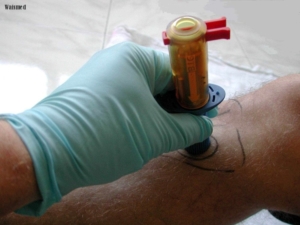
Currently in use (2007), the Adult B.I.G.deploys a 15G catheter, the Pediatric B.I.G. an 18G catheter, into eight different locations (bilaterally – the tibial tuberosity, anterior head of the humerus, medial malleolus, and/or distal metaphysis of the radius). According to the literature, it does so without “shattering” or “splintering” the bone upon entry. The depth can be manually set according to choice of site, ranging from 0.5 cm to 1.5 cm for infants and children, as well as 1.5 cm to 2.5 cm for adults.


The B.I.G. does not require direct access to the marrow chamber, rather it infuses through the Trabecular space in the epiphysis. According to documented studies (offered in print and on the website), there have been no reported cases of interruption to the normal growth process in pediatric patients who have had this device placed, as is a risk clearly delineated with older IO technology.
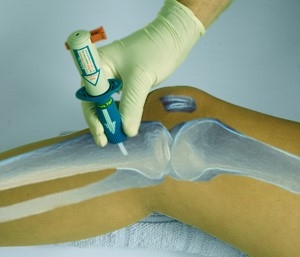
The B.I.G. is suitable for IO access within seconds in the emergent setting, can remain in place for up to six days, and will deliver all therapies normally associated with IV cannulation of the cardiovascular spaces, including whole blood and drug administration. It has also been used to aspirate bone marrow, as needed.
The B.I.G. is in active use by many military special operations teams globally, and has been successfully trialed in hospital mass-casualty settings involving bio-hazard release and can be deployed directly through Level 5 suits.
Submitted to NEMSM May 2007 by K.P. Rickey, images from waismed.com
